A few years ago, I was experimenting with various ways to increase the acidity of sourdough bread and found that I needed a way to produce levains that were similarly mature but at various hydration levels, including some as high as 250%. The “normal” method was to watch for the volume of the levain to rise and when it began to fall back it was declared to be “mature”. But for high hydration mixes, there was not any rising and falling because it was simply too liquid to retain enough CO2 to allow it to increase in volume (other than producing some surface foam which did not seem to be very useful).
After thinking about this for a while, I wondered if there was enough CO2 escaping from the levain to measure the weight that was lost in the process. To find out if there was enough being produced, I did a rough calculation based on the fermentation of glucose to ethyl alcohol and
C6H12O6 → 2 C2H5OH + 2 CO2
One mol of glucose weighs 180g and is converted into 2 mols of ethyl alcohol (46g/mol) and 2 mols of CO2 (44g/mol), so in the process 88g of CO2 is produced of which some escapes and the rest either remains dissolved in the liquid phase of the dough or is retained as gas in the bubbles of the dough. When a levain is mixed, the amylase enzymes in the flour begin to break down some of the starch in the flour (which starts with a just a little maltose and some broken starch granules and after about 6 hours has as much as 6% maltose along with some other fermentable polysaccharides). [Saunders, Ng, and Kline] And the enzymes are recycled so the process of starch degradation continues for as long as there is broken starch for it to work on and the rest of the required conditions are met. So, if we take 10g of flour and after getting it wet and letting the enzymes do their thing for 6 hours, it contains about 600mg of maltose, and because maltose is made up of two glucose molecules, we have 600mg of glucose equivalent. If the formula above held true, about 48% of the weight of the 600mg glucose should show up as CO2. This would yield something like 293mg of CO2, and that should be measurable but would require a high resolution/high accuracy scale.
So, the initial estimate of how much CO2 might be lost was high enough to make it interesting to pursue measuring actual weight loss in a high hydration levain. My expectation was that the amylase enzymes would continue to produce sugars from the starch and the process would run until something (perhaps metabolic byproducts or pH sensitivity might poison the environment) slowed it down.
The next question was what else might be going on that could look like CO2 loss. The first guess was that evaporation of water off the levain surface might be high enough to be a problem, and to address that I ran a simple experiment, measuring the weight of a container of water (about 36g of water in a 4g polypropylene food service cup with a snap-on but not gas-tight) lid in place) over a few days to see if it lost enough weight to get in the way of seeing the loss of CO2.

As you can see, the fluctuation in the weight of the water at refrigerator temperature (38°F) averages to be a very small number, with measured weight differences of less than 20mg over multiple hours when temperature variations may have affected scale accuracy. Once the water was allowed to return to room temperature, evaporation became measurable, losing about 4% of the weight of the water over 15 days or ~0.25% per day. So, it is clear that evaporation is a measurable quantity but when it is refrigerated and the vapor pressure is low, the loss rate is effectively zero.
Now, how much weight does a levain lose over a refresh cycle? And how does that compare with water evaporation? To measure the weight loss on a consistent basis, I use the weight of the added flour to normalize the weight lost to CO2, so for a refresh cycle that starts with 6g of starter, adds 12g of water and 12g of flour, the weight loss is divided by the 12g of added flour to arrive at a percentage that grows with time. If we use the 0.25% per day weight loss due to evaporation and assume (a conservative assumption) that the evaporation of water from the mix will be the same as from a container of pure water and that it will lose 0.25% of the weight of the water over 24 hrs, the weight loss looks like this:
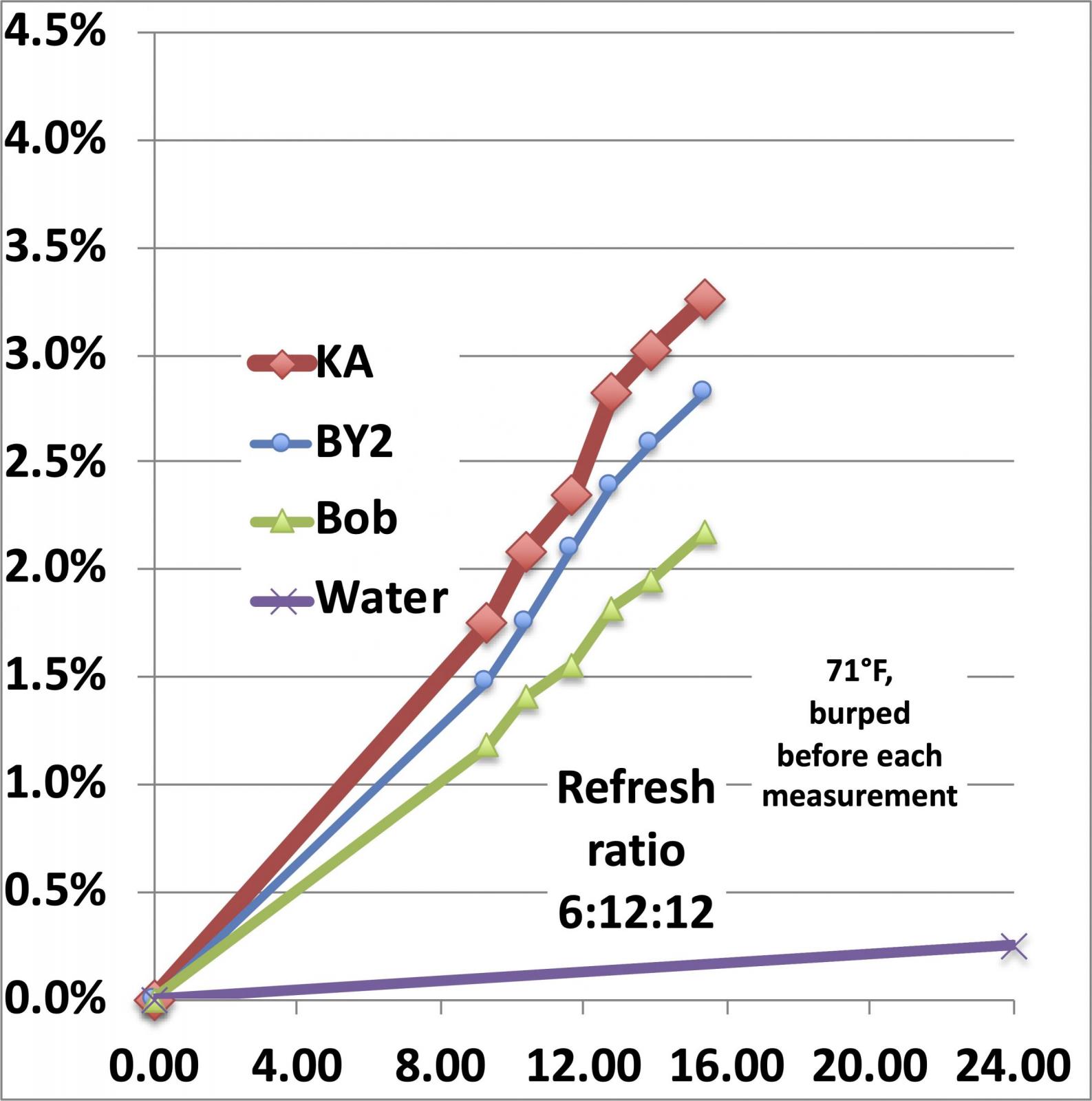
The different starters each exhibits its own weight loss because each one is growing and giving off CO2 at a different rate and in this case, I have plotted a line at the bottom that models the evaporative loss. Thus, the weight loss of an actively growing starter is large enough and fast enough that we don’t have to worry about mistaking water evaporation for CO2 loss. And we can differentiate between the growth rates of different starters (which doesn’t tell us much more than perhaps something about the numerical density of living yeast cells in the seed starter (which sets the initial growth rate).
If the growing starter is refrigerated at any point during the growth cycle, growth effectively stops (it does continue to grow but very slowly and we will see how fast it continues to grow a little later).
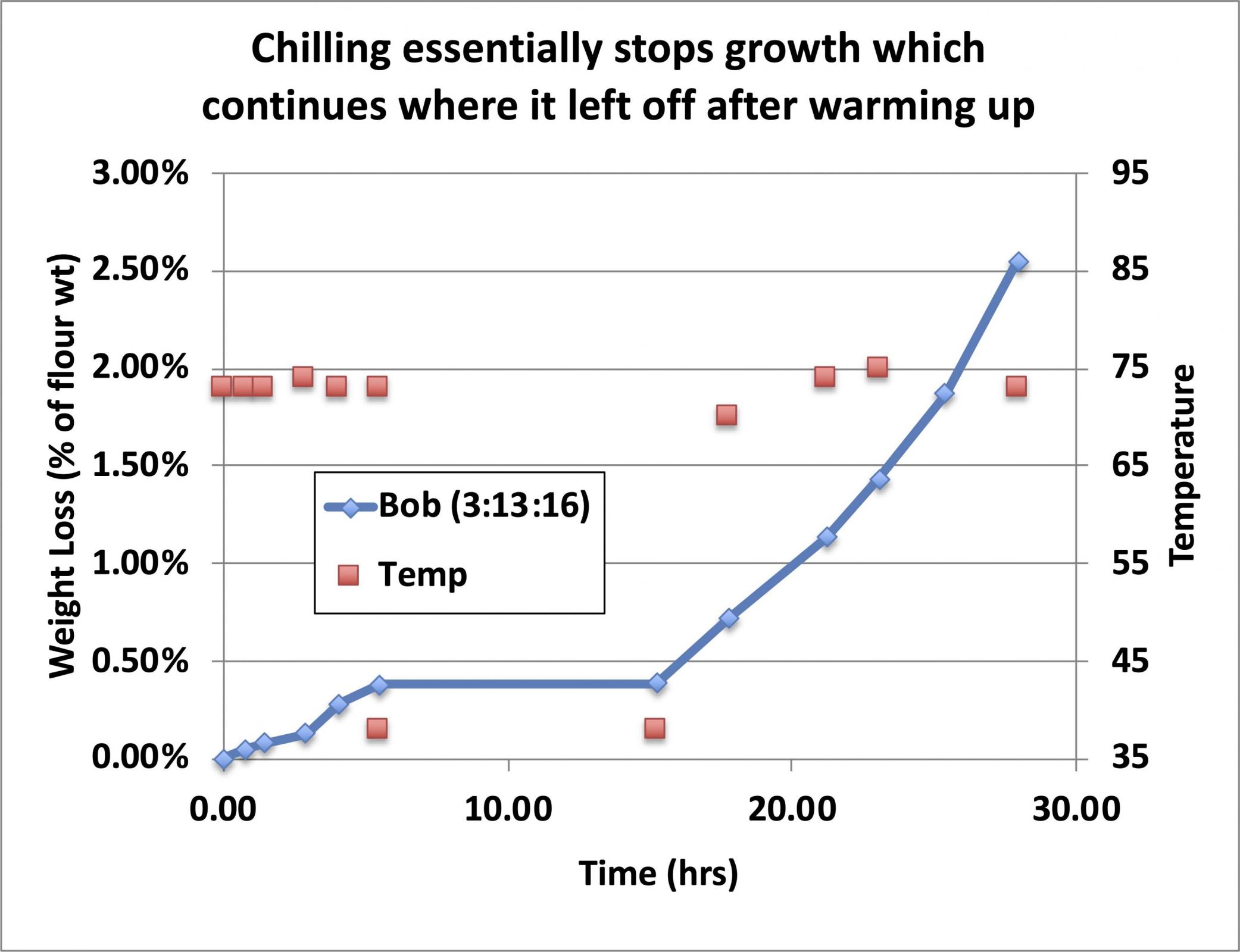
My observation has been that from the appearance of the rise and fall of the starter as it matures, the point at which it begins to fall is generally at the point where it has lost about 2% of the weight of the added flour, so I use this as a guide to judge when a starter is ready to use, even when I can’t tell whether it has begun to fall (perhaps because it rose up and contacted the cover of the container and I thus can’t tell if it fell because of that, or because it was of such high hydration that there is no bulk volume expansion of the growing starter, just some foam floating on the surface of the liquid. When it has lost 2% of the weight of the added flour, it is (by definition) ready to use. It works for me. If you want to use a different number, feel free. “Trial and success” is the name of the game.
Now let’s look at how long you can leave a starter in the refrigerator before you need to feed it. For that experiment I didn’t let the starter get going before I refrigerated it, just mixed it, capped it, and stuck it in the refrigerator. And they were mixed stiff, using a refresh ratio of 5:10:15.
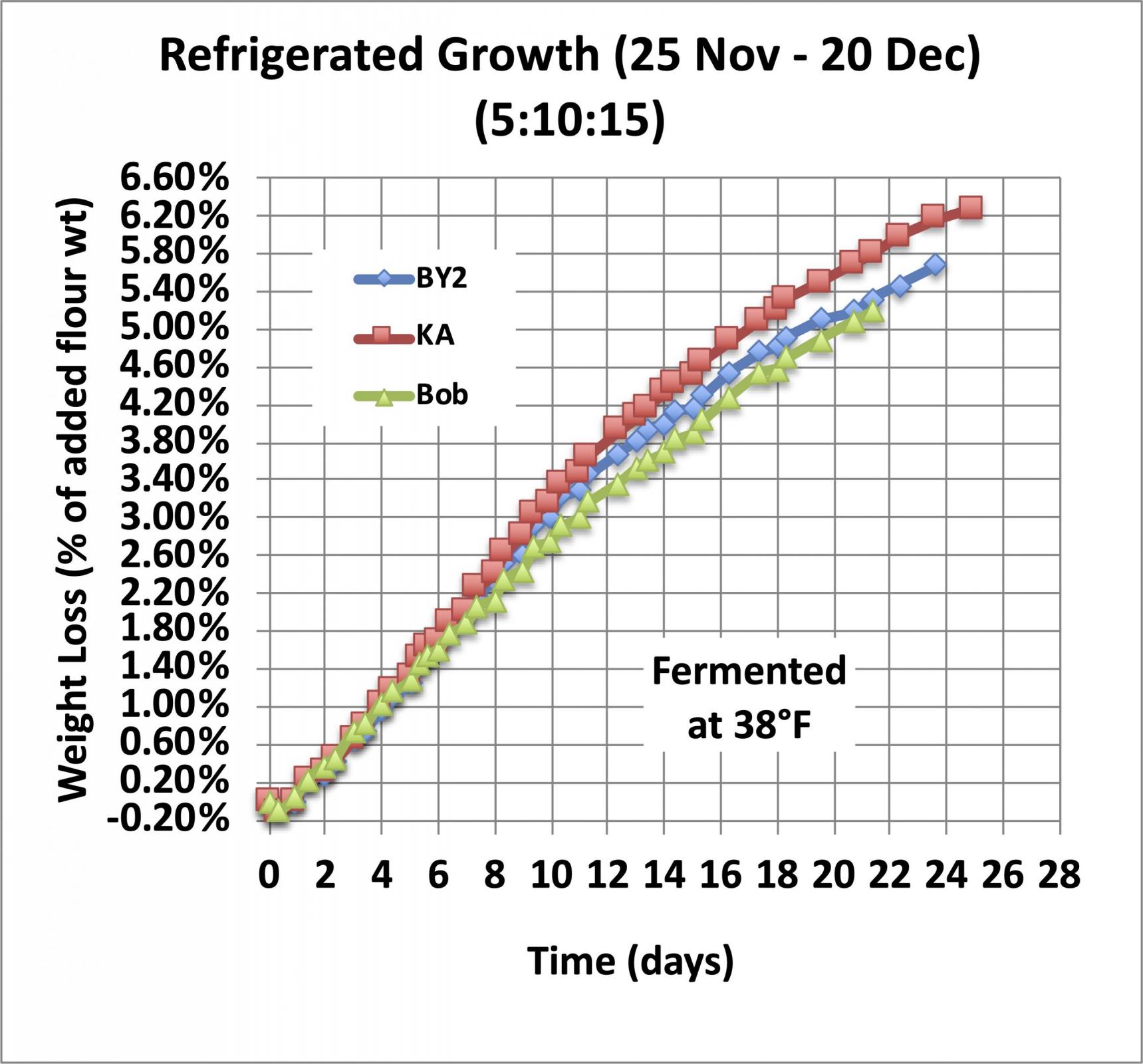
As you can see, in the refrigerator at 38°F, it takes about a week to lose 2% of the weight of the added flour, so if you don’t let your starter grow before you refrigerate it, it will take a week to mature but you can use it without feeding it again.
By day 14, there is some small divergence between the three starters in this test, but the growth rate is still fairly constant (linear growth) for all of them, and I have found that I can still use it to start a levain without an intermediate feeding.
By the end of the third week, there is additional divergence between the three samples shown here, and the weight loss curve is clearly beginning to flatten out, but there is still a significant amount of CO2 being produced. I find that after three weeks I get better performance if I do a double refresh before making a levain. I take this as strong evidence that the native amylase enzymes remain active and continue to convert broken starch into maltose, and the yeast continues to convert the maltose into CO2 and other metabolic products until something limits the process.
Now while all of these experiments demonstrate that weight loss is an adequate method for judging the maturity of starter, it is equally good for gaging the maturity of levain, and it has the advantage that you don’t need a milligram scale to use it. For any levain where you are adding at least 100g of flour and assuming that you have a digital scale that is accurate to 1g, you just cover the bowl of levain with plastic wrap and weigh the bowl, levain, and plastic wrap, and make note of the total weight of the combination, calculate 2% of the weight of the added flour and subtract it from the total and that becomes your target weight for the bowl of starter when the levain is mature. And from the extended cold propagation experiment we know that you can lose as much as 4% of the weight of the added flour and it doesn’t make much difference in terms of the health or proofing capacity of the levain.
Notes:
For stiff starters, there is less water in which the CO2 can dissolve, and any dissolved CO2 will not escape, plus CO2 will be trapped in the alveoli of the expanding starter. In all cases, there is little or no CO2 released until the starter is saturated with CO2 at which point it cannot hold any more. So, when you plot weight loss, expect for there to be a lag between when you mix the starter and when it starts to lose weight. Part of that is due to not having a lot of yeast cells actively consuming sugar and making CO2 soon after being mixed, and part is due to the fact that the early CO2 is being absorbed by the liquid in the starter as well as being stored in internal alveoli (bubbles) within the starter.
While both mechanisms are operating, you need a way to get accurate measurements, and I found that if I would thump (burp) the container on a towel or my hand, it would deflate the bubbles in the starter releasing trapped CO2 and knocking it down to some common (low) level of porosity. If/when I did not do this, the weight loss data was very noisy since the starter will deflate on its own after a period of time and you can’t control it and probably don’t even observe it (it looks like surface bubbles popping but it gives off CO2 which impacts what you weigh). It is also important to remove the lid of the container and blow out any accumulated CO2 that is trapped in the head space between the top of the starter and the top of the container. While CO2 is a gas, it is a heavy gas, and it is measurable and it contributes to measurement noise if you don’t flush it out. Note that larger containers trap more CO2 and the difference in buoyancy is the difference between the molecular weights (at sea level that is 44g per 22.4 liters for CO2, and 29g per 22.4 liters for air).
Fifteen grams per 22,400 ml is 15 mg per 22.4 ml, and half of a 5.5 oz polypropylene food service cup (78 ml) filled with CO2 instead of air adds about 53mg to the measured weight if you don’t replace it with air before measuring. You can do the same calculation for large bowl filled with levain and covered with StretchTite; there is a substantial amount of trapped CO2 and you need to flush it out before you weigh the starter when determining if it is mature.













































