
The 2% weight loss method for judging levain maturity
A few years ago, I was experimenting with various ways to increase the acidity of sourdough bread and found that I needed a way to produce levains that were similarly mature but at various hydration levels, including some as high as 250%. The “normal” method was to watch for the volume of the levain to rise and when it began to fall back it was declared to be “mature”. But for high hydration mixes, there was not any rising and falling because it was simply too liquid to retain enough CO2 to allow it to increase in volume (other than producing some surface foam which did not seem to be very useful).
After thinking about this for a while, I wondered if there was enough CO2 escaping from the levain to measure the weight that was lost in the process. To find out if there was enough being produced, I did a rough calculation based on the fermentation of glucose to ethyl alcohol and
C6H12O6 → 2 C2H5OH + 2 CO2
One mol of glucose weighs 180g and is converted into 2 mols of ethyl alcohol (46g/mol) and 2 mols of CO2 (44g/mol), so in the process 88g of CO2 is produced of which some escapes and the rest either remains dissolved in the liquid phase of the dough or is retained as gas in the bubbles of the dough. When a levain is mixed, the amylase enzymes in the flour begin to break down some of the starch in the flour (which starts with a just a little maltose and some broken starch granules and after about 6 hours has as much as 6% maltose along with some other fermentable polysaccharides). [Saunders, Ng, and Kline] And the enzymes are recycled so the process of starch degradation continues for as long as there is broken starch for it to work on and the rest of the required conditions are met. So, if we take 10g of flour and after getting it wet and letting the enzymes do their thing for 6 hours, it contains about 600mg of maltose, and because maltose is made up of two glucose molecules, we have 600mg of glucose equivalent. If the formula above held true, about 48% of the weight of the 600mg glucose should show up as CO2. This would yield something like 293mg of CO2, and that should be measurable but would require a high resolution/high accuracy scale.
So, the initial estimate of how much CO2 might be lost was high enough to make it interesting to pursue measuring actual weight loss in a high hydration levain. My expectation was that the amylase enzymes would continue to produce sugars from the starch and the process would run until something (perhaps metabolic byproducts or pH sensitivity might poison the environment) slowed it down.
The next question was what else might be going on that could look like CO2 loss. The first guess was that evaporation of water off the levain surface might be high enough to be a problem, and to address that I ran a simple experiment, measuring the weight of a container of water (about 36g of water in a 4g polypropylene food service cup with a snap-on but not gas-tight) lid in place) over a few days to see if it lost enough weight to get in the way of seeing the loss of CO2.

As you can see, the fluctuation in the weight of the water at refrigerator temperature (38°F) averages to be a very small number, with measured weight differences of less than 20mg over multiple hours when temperature variations may have affected scale accuracy. Once the water was allowed to return to room temperature, evaporation became measurable, losing about 4% of the weight of the water over 15 days or ~0.25% per day. So, it is clear that evaporation is a measurable quantity but when it is refrigerated and the vapor pressure is low, the loss rate is effectively zero.
Now, how much weight does a levain lose over a refresh cycle? And how does that compare with water evaporation? To measure the weight loss on a consistent basis, I use the weight of the added flour to normalize the weight lost to CO2, so for a refresh cycle that starts with 6g of starter, adds 12g of water and 12g of flour, the weight loss is divided by the 12g of added flour to arrive at a percentage that grows with time. If we use the 0.25% per day weight loss due to evaporation and assume (a conservative assumption) that the evaporation of water from the mix will be the same as from a container of pure water and that it will lose 0.25% of the weight of the water over 24 hrs, the weight loss looks like this: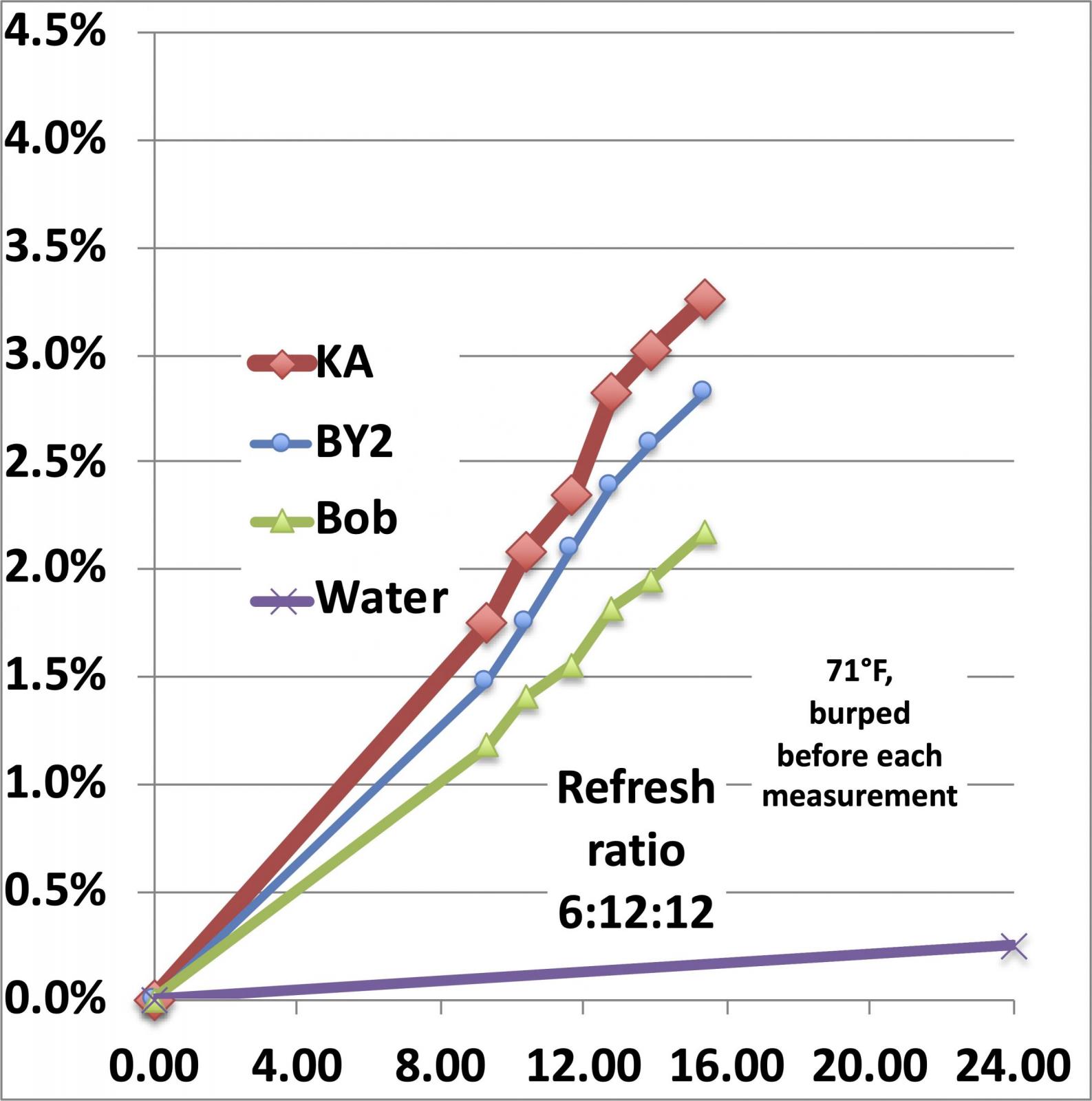
The different starters each exhibits its own weight loss because each one is growing and giving off CO2 at a different rate and in this case, I have plotted a line at the bottom that models the evaporative loss. Thus, the weight loss of an actively growing starter is large enough and fast enough that we don’t have to worry about mistaking water evaporation for CO2 loss. And we can differentiate between the growth rates of different starters (which doesn’t tell us much more than perhaps something about the numerical density of living yeast cells in the seed starter (which sets the initial growth rate).
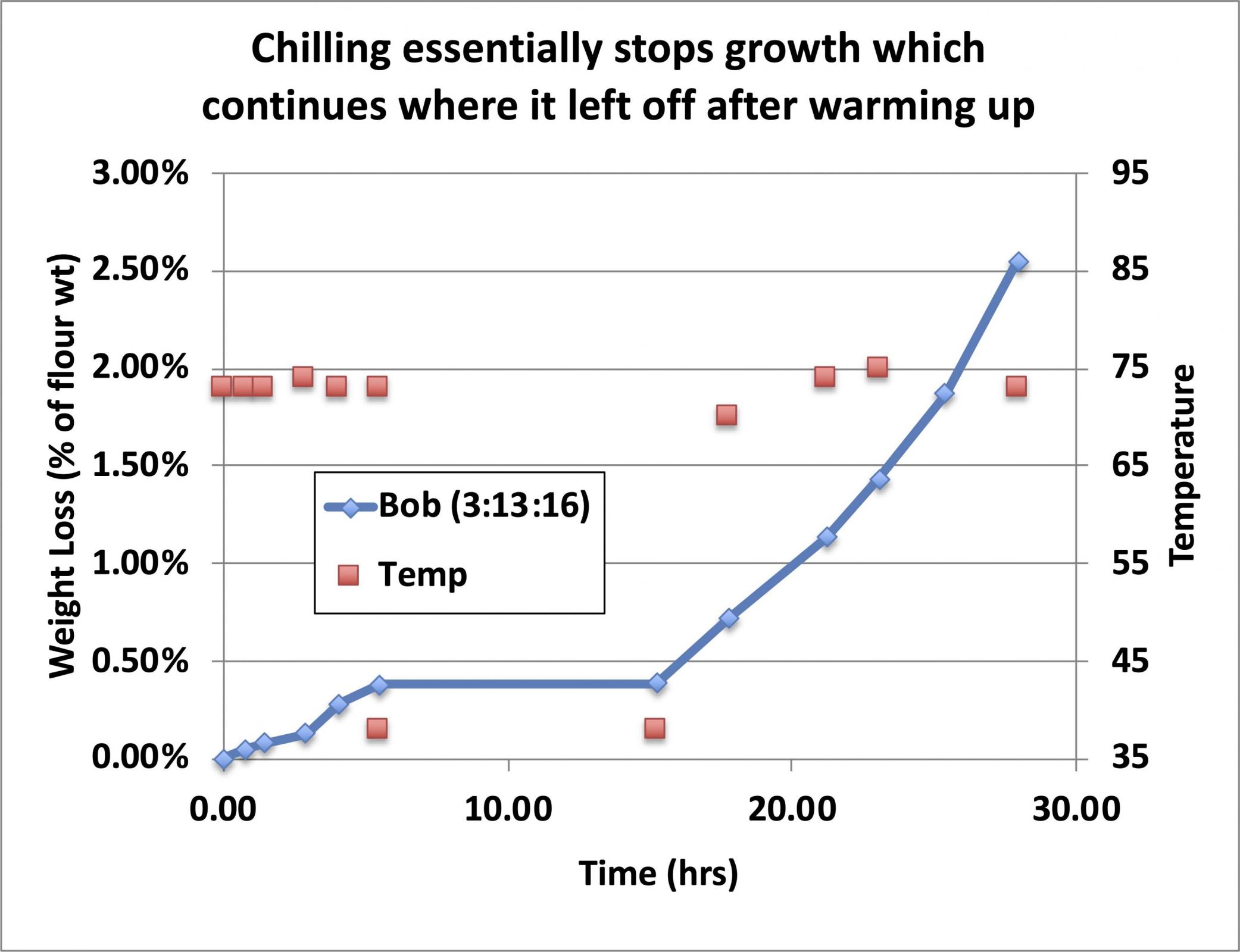
If the growing starter is refrigerated at any point during the growth cycle, growth effectively stops (it does continue to grow but very slowly and we will see how fast it continues to grow a little later).
My observation has been that from the appearance of the rise and fall of the starter as it matures, the point at which it begins to fall is generally at the point where it has lost about 2% of the weight of the added flour, so I use this as a guide to judge when a starter is ready to use, even when I can’t tell whether it has begun to fall (perhaps because it rose up and contacted the cover of the container and I thus can’t tell if it fell because of that, or because it was of such high hydration that there is no bulk volume expansion of the growing starter, just some foam floating on the surface of the liquid. When it has lost 2% of the weight of the added flour, it is (by definition) ready to use. It works for me. If you want to use a different number, feel free. “Trial and success” is the name of the game.
Now let’s look at how long you can leave a starter in the refrigerator before you need to feed it. For that experiment I didn’t let the starter get going before I refrigerated it, just mixed it, capped it, and stuck it in the refrigerator. And they were mixed stiff, using a refresh ratio of 5:10:15.
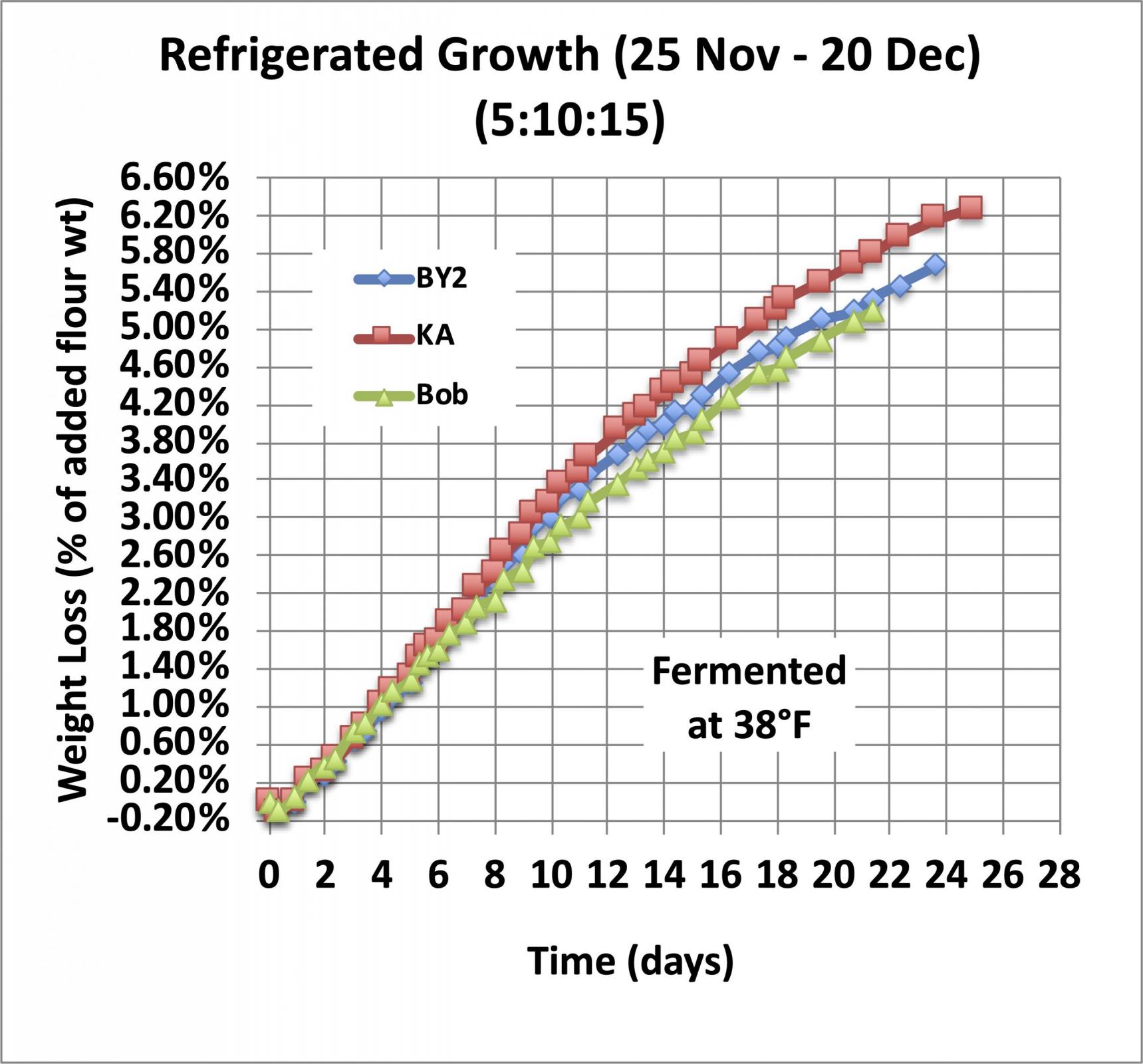
As you can see, in the refrigerator at 38°F, it takes about a week to lose 2% of the weight of the added flour, so if you don’t let your starter grow before you refrigerate it, it will take a week to mature but you can use it without feeding it again.
By day 14, there is some small divergence between the three starters in this test, but the growth rate is still fairly constant (linear growth) for all of them, and I have found that I can still use it to start a levain without an intermediate feeding.
By the end of the third week, there is additional divergence between the three samples shown here, and the weight loss curve is clearly beginning to flatten out, but there is still a significant amount of CO2 being produced. I find that after three weeks I get better performance if I do a double refresh before making a levain. I take this as strong evidence that the native amylase enzymes remain active and continue to convert broken starch into maltose, and the yeast continues to convert the maltose into CO2 and other metabolic products until something limits the process.
Now while all of these experiments demonstrate that weight loss is an adequate method for judging the maturity of starter, it is equally good for gaging the maturity of levain, and it has the advantage that you don’t need a milligram scale to use it. For any levain where you are adding at least 100g of flour and assuming that you have a digital scale that is accurate to 1g, you just cover the bowl of levain with plastic wrap and weigh the bowl, levain, and plastic wrap, and make note of the total weight of the combination, calculate 2% of the weight of the added flour and subtract it from the total and that becomes your target weight for the bowl of starter when the levain is mature. And from the extended cold propagation experiment we know that you can lose as much as 4% of the weight of the added flour and it doesn’t make much difference in terms of the health or proofing capacity of the levain.
Notes:
For stiff starters, there is less water in which the CO2 can dissolve, and any dissolved CO2 will not escape, plus CO2 will be trapped in the alveoli of the expanding starter. In all cases, there is little or no CO2 released until the starter is saturated with CO2 at which point it cannot hold any more. So, when you plot weight loss, expect for there to be a lag between when you mix the starter and when it starts to lose weight. Part of that is due to not having a lot of yeast cells actively consuming sugar and making CO2 soon after being mixed, and part is due to the fact that the early CO2 is being absorbed by the liquid in the starter as well as being stored in internal alveoli (bubbles) within the starter.
While both mechanisms are operating, you need a way to get accurate measurements, and I found that if I would thump (burp) the container on a towel or my hand, it would deflate the bubbles in the starter releasing trapped CO2 and knocking it down to some common (low) level of porosity. If/when I did not do this, the weight loss data was very noisy since the starter will deflate on its own after a period of time and you can’t control it and probably don’t even observe it (it looks like surface bubbles popping but it gives off CO2 which impacts what you weigh). It is also important to remove the lid of the container and blow out any accumulated CO2 that is trapped in the head space between the top of the starter and the top of the container. While CO2 is a gas, it is a heavy gas, and it is measurable and it contributes to measurement noise if you don’t flush it out. Note that larger containers trap more CO2 and the difference in buoyancy is the difference between the molecular weights (at sea level that is 44g per 22.4 liters for CO2, and 29g per 22.4 liters for air).
Fifteen grams per 22,400 ml is 15 mg per 22.4 ml, and half of a 5.5 oz polypropylene food service cup (78 ml) filled with CO2 instead of air adds about 53mg to the measured weight if you don’t replace it with air before measuring. You can do the same calculation for large bowl filled with levain and covered with StretchTite; there is a substantial amount of trapped CO2 and you need to flush it out before you weigh the starter when determining if it is mature.


Comments
Doc, most of this is over my head, but in the past we have discussed this at length. I have used this for starters, but plan to give it a try with the levain.
A 250% hydrated culture also sounds interesting.
The rationale for trying a very liquid levain was to allow the LAB to produce more acid before the acidity (mol of lactate + acetate per liter of liquid) began to slow things down. While we know that LAB replication slows down when the pH gets below ~3.8, Gänzle [Modeling of Growth of Lactobacillus sanfranciscensis and Candida milleri in Response to Process Parameters of Sourdough Fermentation- Michael G. Ganzle, Michael A Ehmann, and Walter P. Hammes] showed that both LAB and yeast activity decline as the concentration of lactate + acetate increases (his experimental method did not allow him to distinguish between acetate and lactate as the source of the sensitivity). So, adding more water to the levain has the effect of diluting the concentration of the undissociated acids and relieving some pressure on both LAB and yeast growth rates and LAB production of acids (which continues even after the reproduction slows or halts due to pH decline below 3.8). The result is that the levain carries more acidity to the dough when the levain is fermented at high hydration. More acid in the levain leads to more acid in the loaf (independent of other avenues to enhance acidity such as adding fructose to the dough).
Doc this is great information, I have heard you talk about 2% weight loss and now I better understand how you came about finding this measure. Also I didn’t realize that you were referring to 2% weight loss of the flour only, but that makes sense of course. Thanks for sharing this very detailed description of your experiments.
Just a big “well done!” from me. Chemistry was never my forte, but as a scientist and long time bread baker I followed it from start to finish. I had never thought about my levain in “molar” terms, but I’m anxious to give this a try! Great work!
Thanks!
- Greg
The data has been sitting in my file for a number of years and Danny finally got me to properly document it.
I love it!
It reminds me that many years ago I built a controller for the CO2 injection in my aquarium based the color change in a small bottle of pH reagent separated from the aquarium water by a small air gap. I wonder if I could track levain maturity with a similar method?
Very interesting read. Thanks.
That is a clever approach to controlling dissolved CO2, but I am not sure it would do what you want in monitoring a levain fermentation. I would be interested in what indicator you chose to tell you that you needed to make an adjustment. You are measuring pH and not CO2 concentration which while related is not proportional. I suspect that you would see pH quickly drop as CO2 approaches saturation in the levain, but from there the LAB are producing lactate which drives the pH down further and is then unrelated to CO2 concentration (though you can assume that the liquid phase of the levain is saturated with CO2 as soon as you observe any bubbles).
I agree that it is unlikely to be a useful indication for monitoring levain fermentation. At best I think you could say something about rate of CO2 production. For controlling CO2 injection all I needed was a monotonic signal, the nonlinearity didn't matter. I had a green LED on one side and a photoresister on the other side; as the reagent changed color the amount of green light passing through it changed.
Sounds like bromothymol blue. And you got by without a filter. That is good to know. Thanks.
“ As you can see, in the refrigerator at 38°F, it takes about a week to lose 2% of the weight of the added flour, so if you don’t let your starter grow before you refrigerate it, it will take a week to mature but you can use it without feeding it again.”
When seeking optimum culture strength, do you advocate immediate retarding after refresh or some length the room temp fermentation before cold storage?
Dan, one problem I see with feeding and immediate cold storage is that your starter wouldn’t be ready for a week and thus you couldn’t bake until at least a week later. That being said I suppose you could take your starter out and allow it to complete fermentation.
I think based on Doc’s findings above, you can fully ferment your starter, then store it in the fridge for a week and make levains during the week without the need for extra refreshment steps.
Doc correct me if that is incorrect.
I let it lose 2% before refrigerating. No claim is made about 38°F being optimum. It is what it is.
My weekly refresh of the retarded starter took place at 8am this morning. It was fed 20:20:20, so 2% of 20 is 0.40g. The culture, including the glass, plastic cover and rubber band weighed 255.35g. It generally matures in 4-4.5 hr @80F. After 4 hr 20 min the starter had just begun to recede. The starter was burped and the cover was opened, then replaced. The weight was 254.94. A loss of 0.41g.
On a number of occasions this method has been used on starters. I am excited to try this with a levain...
Doc, have you considered this concept for completion of BF?
Instead of weighing the entire dough, maybe just gauge the dough in an aliquot jar.
in that you can't really deflate the dough to measure how much CO2 has been produced. You actually want to retain as much of it as you can arrange, so no I have not seriously considered it. Same thing is true for the dough in the aliquot jar. I am interested in how much CO2 is produced but I measure it by monitoring the volumetric growth of the dough in the aliquot jar.
Mine isn't nearly accurate enough for these measurements.
Gary, I have found that the inexpensive digital scales are actually pretty accurate.
I use THIS MODEL for smaller weights.
I’ve always wondered why I could never quite accurately predict the weight of a “finished” levain and always assumed that my incomplete scrapings and the fermentation process itself was causing enough weight loss to account for the less than perfect target levain weight.. So now we have a rule of thumb that lets us monitor levain readiness which is even better than knowing what happened to the angel’s share. Thanks for the deep dive, doc.
Phil
I have it cranked in to my design spreadsheet. And the net dough weight comes in within a few grams.
Hi,
I read a post / thread from you a while back re: whole grains and acid production that was fascinating and wanted to follow up. Basically with some questions.
Is buffering capacity increased with more whole grain dough and lower hydration dough? I believe so. And if this is case, if I want a more sour tasting loaf, I would increase whole grains and/or lower hydration of dough?
all else equal, does temp of initial bulk affect sourness of final loaf? Say a 4 hr bulk at 80 vs a 7 hr bulk at 70?
What about the amount of prefermented flour? Say 30% and shorter bulk vs 10% and longer bulk? My sense is how mature levain is and how mich whole grain it is has might be more important, although unsure?
A long cold retard increases sourness as well, although I don’t know exactly why? Could you elaborate?
can you affect fermentation quotient by adjusting hydration or temp? I’ve read conflicting reports online (frankly I’ve read conflicting info about all of this stuff online).
are there other process items you would highlight to make a loaf more or less sour?
wow, that’s a lot. Thanks in advance for your help. I greatly appreciate it.
regards,
adam
IG — sourdough_gambit_22
I found that TTA went up as whole wheat flour fraction increased, at least in the 0-20% range. I have no data on what hydration does to TTA, but if you find out I am interested in looking at the data. If you add some fructose to the mix, you have an independent path to increased TTA because the addition of fructose promotes/facilitates a pathway that results in higher acetic acid production.
In mwilson’s post below he points out that "Fructose, a 6 Carbon sugar is not converted to acetic acid, a 2 carbon molecule. Rather fructose is used as an electron acceptor and in this case, fructose is used to regenerate NAD+ by reducing (converting) fructose to mannitol, this in turn favours a pathway which directs the 2 remaining carbons from glucose (from half maltose) (6 carbons minus 3 for lactate and 1 for CO2 (decarboxylation)) toward acetate instead of ethanol and in doing so gaining additional ATP."
Adding sucrose should in theory yield the same result, but when I tried it the result was a less sour loaf. I think this is perhaps a case where commercial yeast makes sufficient invertase to split robust quantities of sucrose into glucose + fructose while some yeasts in sourdough starters may not make as much. That is an easy experiment to run with your starter: two batches, one with 2% fructose and one with 4% sucrose (sucrose is split into glucose + fructose by invertase).
I find that it does, but the time difference is not proportional to the temperature difference. 10 hr bulk @ 2% pre-fermented flour may be equivalent to 12 hr bulk @ 1% pff. Since the growth is approximately exponential, a factor of two in initial conditions is really only one doubling of bacterial population which is as short as 90 min at optimum temperature and without any other drags on reproduction (salt comes to mind).
I have found that 28-30% pff is about as high as I can go without paying too much of a penalty. At the other end of the scale, below ~0.25% it takes quite a while for the LAB to acidify the dough to the point where the growth rate gets close to optimum (pH~5.5) and you have to add a lot of time waiting for the real exponential growth to start. I generally run in the range from 1% to 30%; 1%, warm, and long for a more sour result, 28% and warm if I am in a hurry.
I have seen this result occasionally but I don't go there on a habitual basis. My guess is that at low temperatures the yeast and LAB growth rates while low are not zero and the LAB continues to grow and produce acid after the yeast shuts down (that is a very high growth rate ratio). But if you get too cold the LAB shuts down too so you get no effect. It is a zone that merits further experimentation but the instrumentation will be tricky and the DOE has a lot of degrees of freedom so it needs lots of cycles to get a clear signal.
I have seen the same reports but I have no personal data. I add fructose if I want more acetic acid - works like a charm.
Probably, but there are at least 20 variables and ratios that need to be accounted for, so once you have found a design point that works for you I suspect that you don't need to search the entire space for better solutions. You can, but it may not produce a useful result. Local optimization is perhaps the most productive approach. With 20 variables, you can theoretically hold 19 of the constant and run three experiments to bracket your chosen design point to see what each partial derivative is at that point. That is 60 experiments if your process control and instrumentation are flawless. Now pick which variable you want to change to define a new baseline. Repeat until exhausted.
Thanks so much for quick and thorough response!
I came across this article recently — THE TECHNOLOGICAL EVALUATION OF SOURDOUGHS PREPARED IN DIFFERENT CONDITIONS.
it suggests lower hydration doughs develop more tta. although I still understand why Panettone traditionally uses a very stiff starter and that does not develop any sourness. Anyway …
This is in line with one baker who nerds out on Instagram. His name is Ian Lowe @apieceofbread. Check out some of his threads for some interesting discussion.
thanks again!
Doc, some things to consider…
It is well established that lower hydration amplifies the buffering capacity of flour. Not sure you need an experiment to prove it, as even mild contemplation should deduce what is perhaps obvious. Take for example; if flour is constant but water content is lower (lower hydration) then the buffering components (from flour) are more concentrated relative to the liquid phase, which is where everything microbial happens and importantly being so that pH is the measure of activity of H+ ions in an aqueous solution.
Not quite, you might need to brush up on hetero-lactic fermentation, as you state it, that is not accurate. Fructose, a 6 Carbon sugar is not converted to acetic acid, a 2 carbon molecule. Rather fructose is used as an electron acceptor and in this case, fructose is used to regenerate NAD+ by reducing (converting) fructose to mannitol, this in turn favours a pathway which directs the 2 remaining carbons from glucose (from half maltose) (6 carbons minus 3 for lactate and 1 for CO2 (decarboxylation)) toward acetate instead of ethanol and in doing so gaining additional ATP.
Unless you can provide a reference, I am quite prepared to rubbish this idea. Do you not remember me mentioning the ubiquity of invertase? There is no reason I can think of why sourdough yeasts should be deficient or even missing this enzyme as you suggest.
I am always enlightened when you take the time to comment and I am greatly appreciative that you have done so in this case.
When it comes to measuring TTA, if you remove some of the water and in the process reduce the weight of the sample but do not remove or neutralize any of the acids, then the amount of NaOH required to neutralize the residual acidity will not change. But the sample weight goes down so the TTA goes up. That is clear. What I find not so clear is how much acid is produced in a dough with lower water activity. If it is roughly the same, then your explanation covers it. But I am not well enough educated to judge whether that is the result when the LAB are asked to operate in a more highly concentrated environment.
Your explanation of the process steps that result in higher acetic acid production when fructose in present in the environment is crystal clear, and is greatly appreciated. So fructose is not "converted" to acetic acid but does promote/facilitate a pathway that results in higher acetic acid production. Is there a reference you can point to that lays out the energetics of that pathway and identifies the specifics of what "favours" the production of acetate over ethanol? Having not appreciated the existence of the pathway, I now want to better understand it.
It was suggested to me that acidity could be increased by adding some sucrose to the dough because it would result in fructose being liberated to yield more acetic acid. So being inquisitive, I ran a split batch with 4% sucrose in one leg and 2% fructose in the other leg and no commercial yeast in either half. To my great surprise, the leg with the fructose was dramatically more acidic than the leg with sucrose. When I asked Michael Gäenzle about why, he said that he didn't understand it either, but that commerical yeast has plenty of invertase to break down the sucrose and liberate the fructose, and he didn't know to what extent sourdough-associated yeasts were equally well endowed. So I have an observation and a speculation, but no measurement. If it varies according to the specifics of the yeast in your starter, then just trying it is not a good way to proceed. And I did not do a TTA titration so I don't have quantitative results. So you may be right, but it doesn't change my perception of the one-off result. Do you have any other candidate explanations or suggested experiments to help illuminate what is going on?